lewis structure for argon|Lewis Dot Structure for Argon Atom (Ar) : Tagatay Introduction to Lewis structures. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or . Best Blackjack Apps for Strategy & Card Counting Practice. Being a blackjack expert takes practice. Free blackjack games are good for testing your gameplay with zero risk, but if you want to .
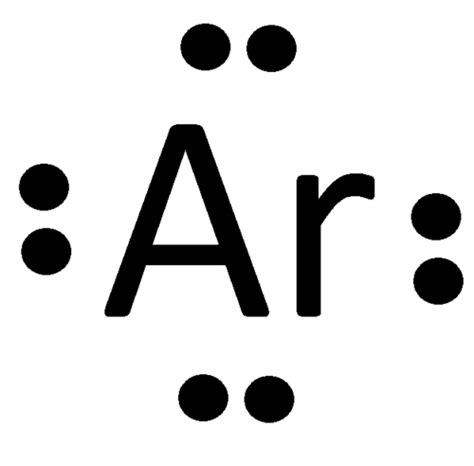
lewis structure for argon,A step-by-step explanation of how to draw the Lewis dot structure for Ar (Argon). I show you where Argon is on the periodic table and how to determine how many valence electrons Argon has..In summary, the Lewis dot diagram for argon shows a total of 8 valence electrons, represented by dots surrounding the symbol of argon. Argon’s electron configuration of .Lewis Structures are visual representations of the bonds between atoms and illustrate the lone pairs of electrons in molecules. They can also be called Lewis dot .
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or .lewis structure for argon Introduction to Lewis structures. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or .The structure of argon can be understood through the Lewis dot diagram, which is a visual representation of the valence electrons. Argon has 18 electrons arranged in three .

The Bohr model of atom defines the atomic structure of elements through a pictorial representation illustrating all the atomic particles viz. electrons, protons, and neutrons. Before we dig deep into .
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis .
A Lewis dot structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of .
In this video we'll look at the atomic structure and Bohr model for the Argon atom (Ar). We’ll use a Bohr diagram to visually represent where the electrons a.In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital.
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order .Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. . However, argon has the electronic structure \(1s^22s^22p^63s^23p^6\), so we can . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below .Draw a Lewis electron dot diagram for any atom or a monatomic ion with an atomic number of less than 20. . A Lewis dot structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. . argon . Solution. a) Phosphorous .English: This is the dot diagram I made for Argon and has 8 dots which represent 8 valence electronlewis structure for argon Lewis Dot Structure for Argon Atom (Ar) Lewis electron dot diagram. The Lewis electron dot diagram (or Lewis structure) is the representation of an atom with its symbol and number of valence electrons around the symbol shown as "dots". The number of dots is equal to number of valence electrons in the atom. The dots take place above and below and left and right position of the Symbol.

A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.3.1 7.3. 1 shows the Lewis symbols for the elements of the third period of the periodic table. Electron dots are typically arranged in four pairs located on the four "sides" of the atomic symbol.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the .
Argon is a noble gas with the atomic symbol Ar, atomic number 18, and atomic weight It is used in fluorescent tubes and wherever an inert atmosphere is desired and nitrogen cannot be used. Electron-Dot .
Exercise 3.15.1 3.15. 1. Draw a Lewis structure that represents the compound that is formed when bromine and phosphorus bond with one another. Answer. 3.15: Covalent Bonding: Drawing Lewis Structures of Covalent Molecules is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.Lewis structure of a water molecule. Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any .Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their valence level. Therefore, these elements have a full valence level that has the . Lewis structure: Formalism used to show the structure of a molecule or compound, in which shared electrons pairs between atoms are indicated by dashes. Non-bonding, lone pairs of .These are all gaseous under normal conditions of temperature and pressure, and are called 'noble gases.' Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their valence level. . Lewis structure: Formalism used to show the structure of a molecule or compound, in which shared electrons pairs between atoms are indicated .Lewis Dot Structure for Argon Atom (Ar) A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol .
The structure of argon can be understood through the Lewis dot diagram, which is a visual representation of the valence electrons. Argon has 18 electrons arranged in three energy levels – two in the first energy level, eight in the second energy level, and eight in the third energy level.
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of .
lewis structure for argon|Lewis Dot Structure for Argon Atom (Ar)
PH0 · The Lewis Dot Diagram: Understanding the Structure of Argon
PH1 · Lewis Structure Finder
PH2 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols
PH3 · Lewis Dot Structure for Argon Atom (Ar)
PH4 · How to Create a Lewis Dot Diagram for Argon: A Step
PH5 · Argon Lewis Dot Structure: 5 Things Beginner's Don't Know!
PH6 · Argon Lewis Dot Structure: 5 Things Beginner's Don't Know!
PH7 · Argon Bohr Model — Diagram, Steps To Draw
PH8 · Argon Bohr Model — Diagram, Steps To Draw
PH9 · 9.3: Drawing Lewis Structures
PH10 · 7.2 Lewis Dot Structures – Introduction to Chemistry
PH11 · 5.1: Lewis Structures